
Question Number 18199 by Tinkutara last updated on 17/Jul/17
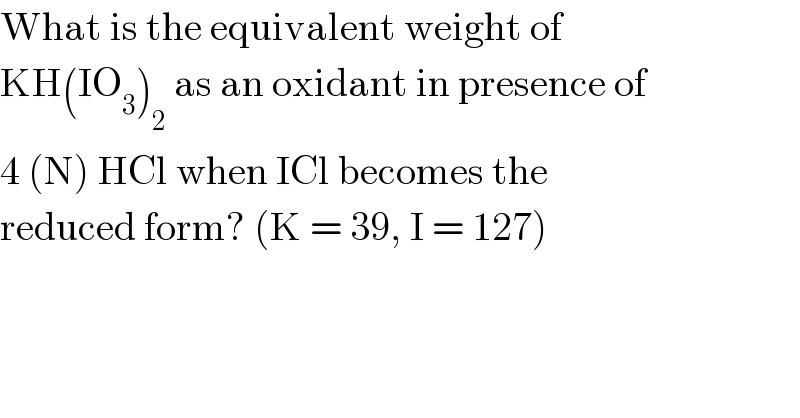
$$\mathrm{What}\:\mathrm{is}\:\mathrm{the}\:\mathrm{equivalent}\:\mathrm{weight}\:\mathrm{of} \\ $$$$\mathrm{KH}\left(\mathrm{IO}_{\mathrm{3}} \right)_{\mathrm{2}} \:\mathrm{as}\:\mathrm{an}\:\mathrm{oxidant}\:\mathrm{in}\:\mathrm{presence}\:\mathrm{of} \\ $$$$\mathrm{4}\:\left(\mathrm{N}\right)\:\mathrm{HCl}\:\mathrm{when}\:\mathrm{ICl}\:\mathrm{becomes}\:\mathrm{the} \\ $$$$\mathrm{reduced}\:\mathrm{form}?\:\left(\mathrm{K}\:=\:\mathrm{39},\:\mathrm{I}\:=\:\mathrm{127}\right) \\ $$
