
Question Number 18202 by Tinkutara last updated on 16/Jul/17

$$\mathrm{An}\:\mathrm{open}\:\mathrm{vessel}\:\mathrm{at}\:\mathrm{27}°\mathrm{C}\:\mathrm{is}\:\mathrm{heated}\:\mathrm{until} \\ $$$$\frac{\mathrm{3}}{\mathrm{5}}\:\mathrm{parts}\:\mathrm{of}\:\mathrm{the}\:\mathrm{air}\:\mathrm{in}\:\mathrm{it}\:\mathrm{has}\:\mathrm{been}\:\mathrm{expelled}. \\ $$$$\mathrm{Assuming}\:\mathrm{that}\:\mathrm{the}\:\mathrm{volume}\:\mathrm{of}\:\mathrm{the}\:\mathrm{vessel} \\ $$$$\mathrm{remains}\:\mathrm{constant},\:\mathrm{find}\:\mathrm{the}\:\mathrm{temperature} \\ $$$$\mathrm{to}\:\mathrm{which}\:\mathrm{the}\:\mathrm{vessel}\:\mathrm{has}\:\mathrm{been}\:\mathrm{heated}. \\ $$
Answered by ajfour last updated on 16/Jul/17
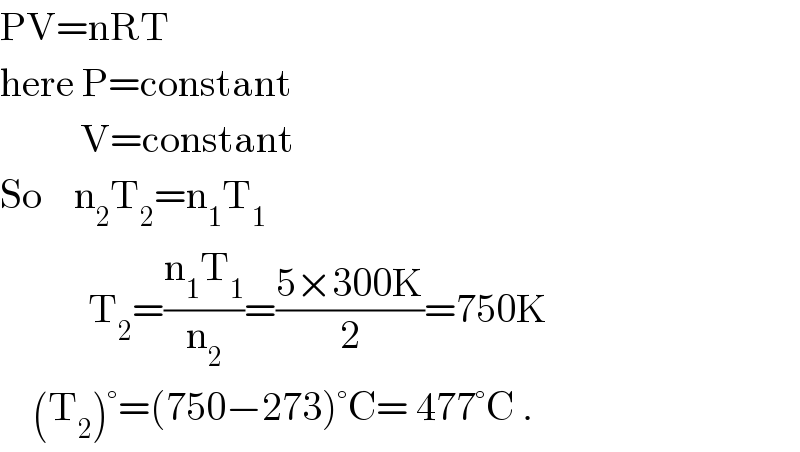
$$\mathrm{PV}=\mathrm{nRT} \\ $$$$\mathrm{here}\:\mathrm{P}=\mathrm{constant} \\ $$$$\:\:\:\:\:\:\:\:\:\:\mathrm{V}=\mathrm{constant} \\ $$$$\mathrm{So}\:\:\:\:\mathrm{n}_{\mathrm{2}} \mathrm{T}_{\mathrm{2}} =\mathrm{n}_{\mathrm{1}} \mathrm{T}_{\mathrm{1}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\mathrm{T}_{\mathrm{2}} =\frac{\mathrm{n}_{\mathrm{1}} \mathrm{T}_{\mathrm{1}} }{\mathrm{n}_{\mathrm{2}} }=\frac{\mathrm{5}×\mathrm{300K}}{\mathrm{2}}=\mathrm{750K} \\ $$$$\:\:\:\:\left(\mathrm{T}_{\mathrm{2}} \right)°=\left(\mathrm{750}−\mathrm{273}\right)°\mathrm{C}=\:\mathrm{477}°\mathrm{C}\:. \\ $$
Commented by Tinkutara last updated on 17/Jul/17

$$\mathrm{Thanks}\:\mathrm{Sir}! \\ $$
