
Question Number 18568 by Tinkutara last updated on 24/Jul/17
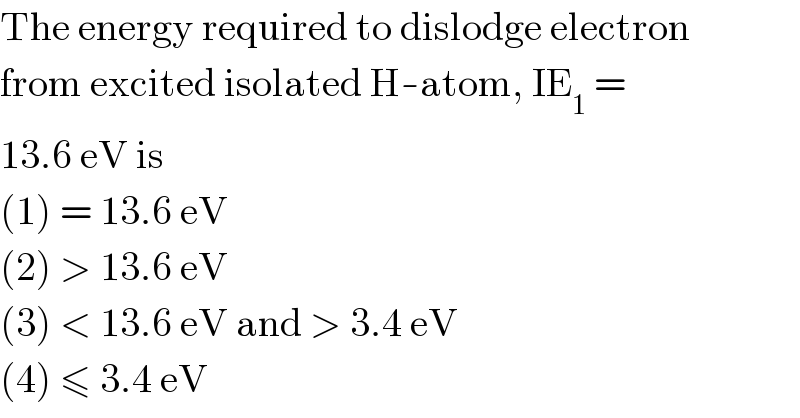
$$\mathrm{The}\:\mathrm{energy}\:\mathrm{required}\:\mathrm{to}\:\mathrm{dislodge}\:\mathrm{electron} \\ $$ $$\mathrm{from}\:\mathrm{excited}\:\mathrm{isolated}\:\mathrm{H}-\mathrm{atom},\:\mathrm{IE}_{\mathrm{1}} \:= \\ $$ $$\mathrm{13}.\mathrm{6}\:\mathrm{eV}\:\mathrm{is} \\ $$ $$\left(\mathrm{1}\right)\:=\:\mathrm{13}.\mathrm{6}\:\mathrm{eV} \\ $$ $$\left(\mathrm{2}\right)\:>\:\mathrm{13}.\mathrm{6}\:\mathrm{eV} \\ $$ $$\left(\mathrm{3}\right)\:<\:\mathrm{13}.\mathrm{6}\:\mathrm{eV}\:\mathrm{and}\:>\:\mathrm{3}.\mathrm{4}\:\mathrm{eV} \\ $$ $$\left(\mathrm{4}\right)\:\leqslant\:\mathrm{3}.\mathrm{4}\:\mathrm{eV} \\ $$
