
Question Number 22447 by Tinkutara last updated on 18/Oct/17
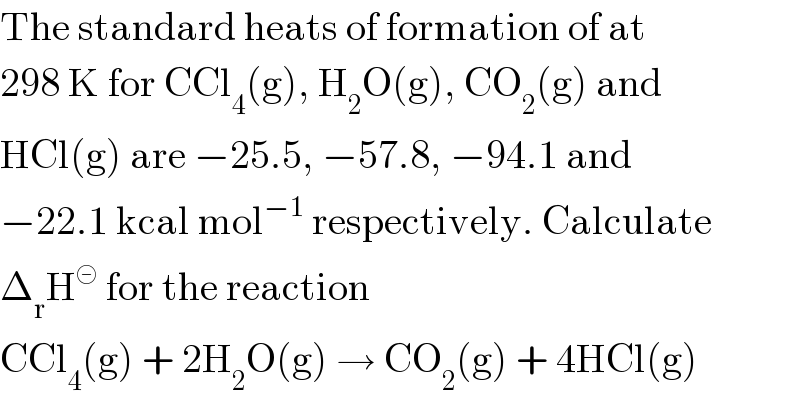
$$\mathrm{The}\:\mathrm{standard}\:\mathrm{heats}\:\mathrm{of}\:\mathrm{formation}\:\mathrm{of}\:\mathrm{at} \\ $$$$\mathrm{298}\:\mathrm{K}\:\mathrm{for}\:\mathrm{CCl}_{\mathrm{4}} \left(\mathrm{g}\right),\:\mathrm{H}_{\mathrm{2}} \mathrm{O}\left(\mathrm{g}\right),\:\mathrm{CO}_{\mathrm{2}} \left(\mathrm{g}\right)\:\mathrm{and} \\ $$$$\mathrm{HCl}\left(\mathrm{g}\right)\:\mathrm{are}\:−\mathrm{25}.\mathrm{5},\:−\mathrm{57}.\mathrm{8},\:−\mathrm{94}.\mathrm{1}\:\mathrm{and} \\ $$$$−\mathrm{22}.\mathrm{1}\:\mathrm{kcal}\:\mathrm{mol}^{−\mathrm{1}} \:\mathrm{respectively}.\:\mathrm{Calculate} \\ $$$$\Delta_{\mathrm{r}} \mathrm{H}^{\circleddash} \:\mathrm{for}\:\mathrm{the}\:\mathrm{reaction} \\ $$$$\mathrm{CCl}_{\mathrm{4}} \left(\mathrm{g}\right)\:+\:\mathrm{2H}_{\mathrm{2}} \mathrm{O}\left(\mathrm{g}\right)\:\rightarrow\:\mathrm{CO}_{\mathrm{2}} \left(\mathrm{g}\right)\:+\:\mathrm{4HCl}\left(\mathrm{g}\right) \\ $$
Answered by Sahib singh last updated on 18/Oct/17
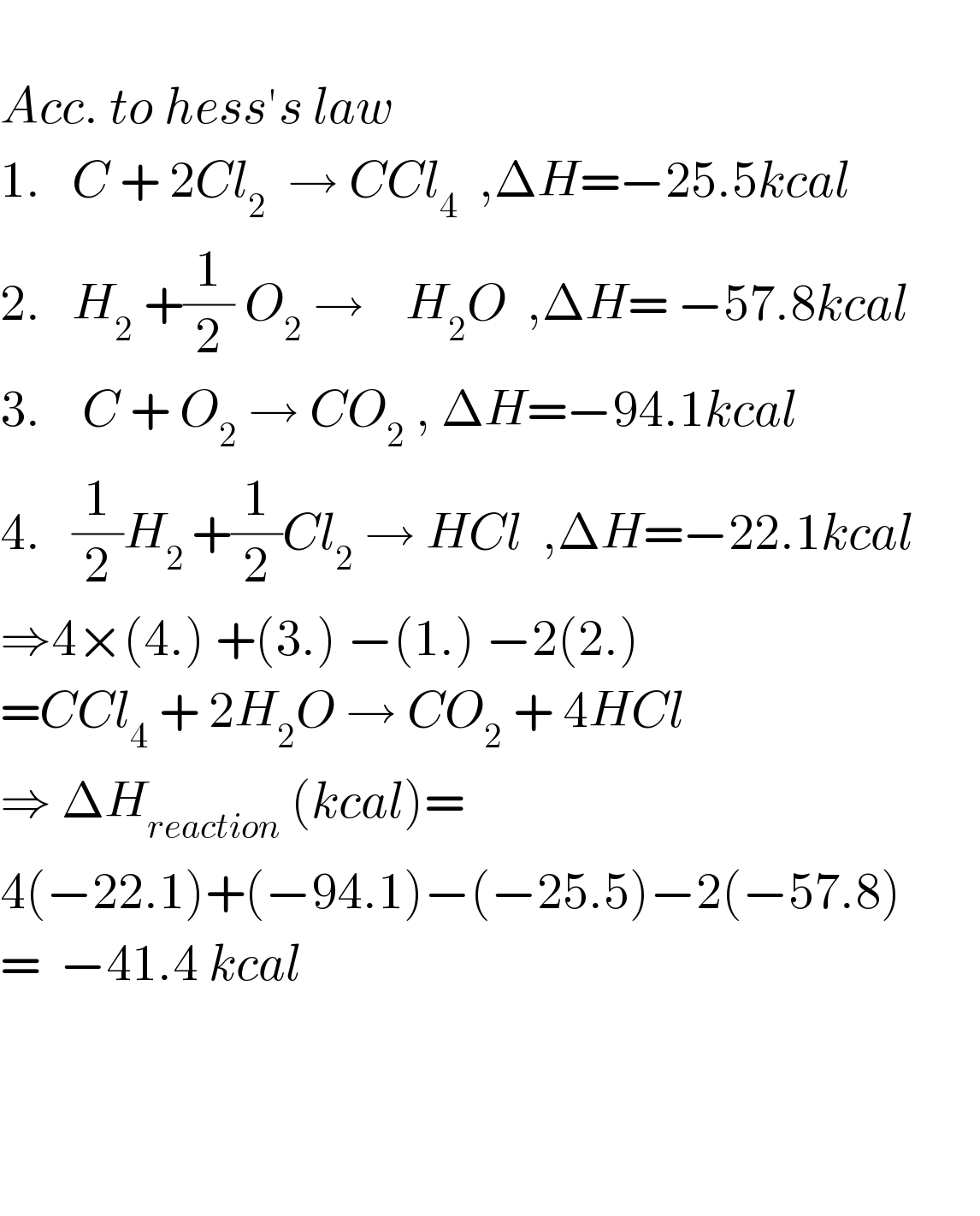
$$ \\ $$$${Acc}.\:{to}\:{hess}'{s}\:{law} \\ $$$$\mathrm{1}.\:\:\:{C}\:+\:\mathrm{2}{Cl}_{\mathrm{2}} \:\:\rightarrow\:{CCl}_{\mathrm{4}} \:\:,\Delta{H}=−\mathrm{25}.\mathrm{5}{kcal}\:\:\:\:\:\: \\ $$$$\mathrm{2}.\:\:\:{H}_{\mathrm{2}} \:+\frac{\mathrm{1}}{\mathrm{2}}\:{O}_{\mathrm{2}} \:\rightarrow\:\:\:\:{H}_{\mathrm{2}} {O}\:\:,\Delta{H}=\:−\mathrm{57}.\mathrm{8}{kcal}\:\:\:\:\:\:\: \\ $$$$\mathrm{3}.\:\:\:\:{C}\:+\:{O}_{\mathrm{2}} \:\rightarrow\:{CO}_{\mathrm{2}} \:,\:\Delta{H}=−\mathrm{94}.\mathrm{1}{kcal}\:\:\:\: \\ $$$$\mathrm{4}.\:\:\:\frac{\mathrm{1}}{\mathrm{2}}{H}_{\mathrm{2}\:} +\frac{\mathrm{1}}{\mathrm{2}}{Cl}_{\mathrm{2}} \:\rightarrow\:{HCl}\:\:,\Delta{H}=−\mathrm{22}.\mathrm{1}{kcal} \\ $$$$\Rightarrow\mathrm{4}×\left(\mathrm{4}.\right)\:+\left(\mathrm{3}.\right)\:−\left(\mathrm{1}.\right)\:−\mathrm{2}\left(\mathrm{2}.\right) \\ $$$$={CCl}_{\mathrm{4}} \:+\:\mathrm{2}{H}_{\mathrm{2}} {O}\:\rightarrow\:{CO}_{\mathrm{2}} \:+\:\mathrm{4}{HCl} \\ $$$$\Rightarrow\:\Delta{H}_{{reaction}} \:\left({kcal}\right)= \\ $$$$\mathrm{4}\left(−\mathrm{22}.\mathrm{1}\right)+\left(−\mathrm{94}.\mathrm{1}\right)−\left(−\mathrm{25}.\mathrm{5}\right)−\mathrm{2}\left(−\mathrm{57}.\mathrm{8}\right) \\ $$$$=\:\:−\mathrm{41}.\mathrm{4}\:{kcal} \\ $$$$ \\ $$$$ \\ $$$$ \\ $$
Commented by Tinkutara last updated on 18/Oct/17

$$\mathrm{Wrong}\:\mathrm{answer}. \\ $$
Commented by Sahib singh last updated on 18/Oct/17

$${Sorry}\:.\:{Just}\:{made} \\ $$$${mistake}\:{in}\:{final} \\ $$$${calculation}. \\ $$
Commented by Tinkutara last updated on 18/Oct/17
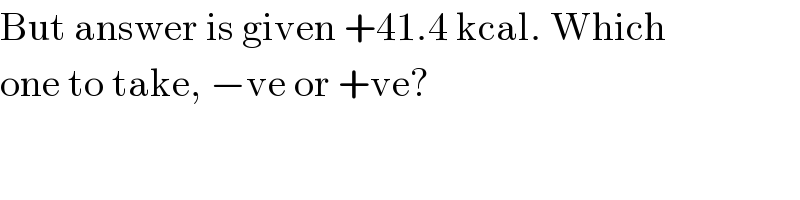
$$\mathrm{But}\:\mathrm{answer}\:\mathrm{is}\:\mathrm{given}\:+\mathrm{41}.\mathrm{4}\:\mathrm{kcal}.\:\mathrm{Which} \\ $$$$\mathrm{one}\:\mathrm{to}\:\mathrm{take},\:−\mathrm{ve}\:\mathrm{or}\:+\mathrm{ve}? \\ $$
Commented by Tinkutara last updated on 20/Oct/17

$$\mathrm{help}\:\mathrm{pls} \\ $$
Commented by Tinkutara last updated on 20/Oct/17
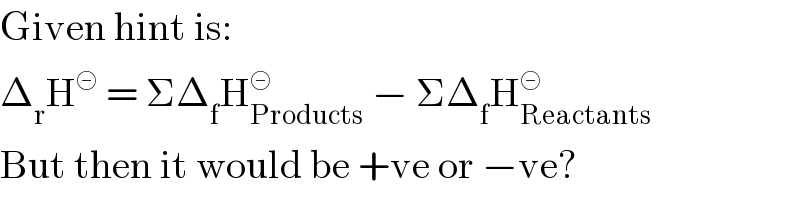
$$\mathrm{Given}\:\mathrm{hint}\:\mathrm{is}: \\ $$$$\Delta_{\mathrm{r}} \mathrm{H}^{\circleddash} \:=\:\Sigma\Delta_{\mathrm{f}} \mathrm{H}_{\mathrm{Products}} ^{\circleddash} \:−\:\Sigma\Delta_{\mathrm{f}} \mathrm{H}_{\mathrm{Reactants}} ^{\circleddash} \\ $$$$\mathrm{But}\:\mathrm{then}\:\mathrm{it}\:\mathrm{would}\:\mathrm{be}\:+\mathrm{ve}\:\mathrm{or}\:−\mathrm{ve}? \\ $$
Commented by Tinkutara last updated on 21/Oct/17
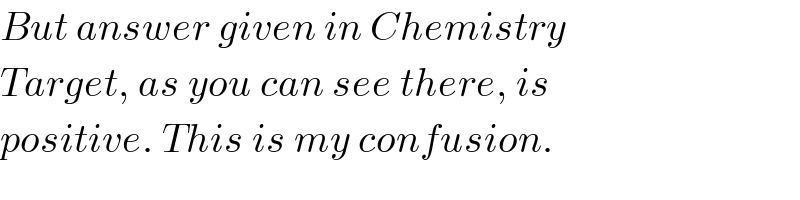
$${But}\:{answer}\:{given}\:{in}\:{Chemistry} \\ $$$${Target},\:{as}\:{you}\:{can}\:{see}\:{there},\:{is} \\ $$$${positive}.\:{This}\:{is}\:{my}\:{confusion}. \\ $$
Commented by Sahib singh last updated on 21/Oct/17
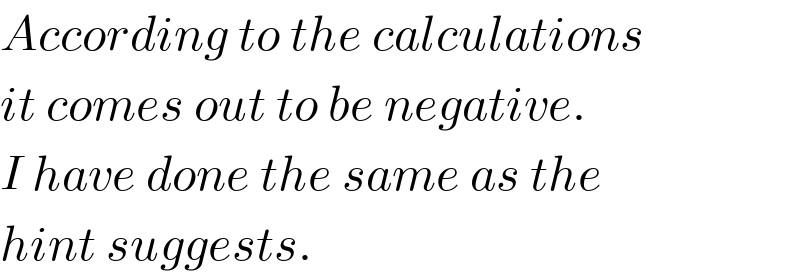
$${According}\:{to}\:{the}\:{calculations} \\ $$$${it}\:{comes}\:{out}\:{to}\:{be}\:{negative}. \\ $$$${I}\:{have}\:{done}\:{the}\:{same}\:{as}\:{the} \\ $$$${hint}\:{suggests}. \\ $$
