
Question Number 23270 by Tinkutara last updated on 28/Oct/17
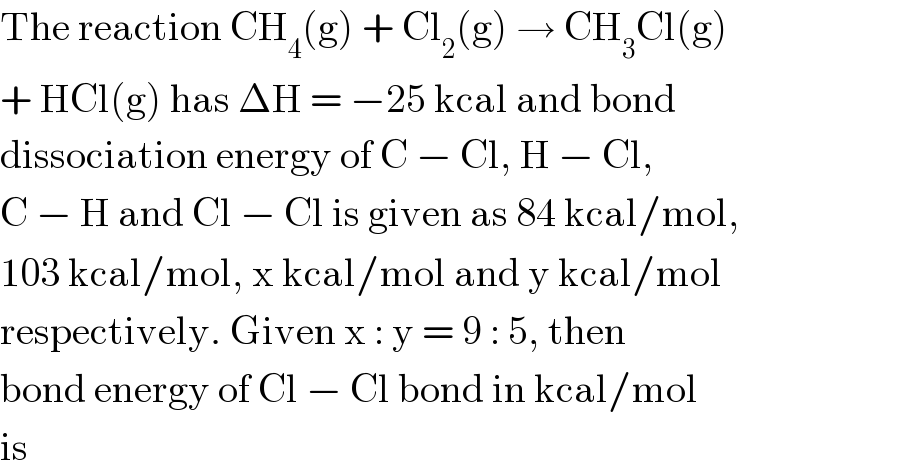
$$\mathrm{The}\:\mathrm{reaction}\:\mathrm{CH}_{\mathrm{4}} \left(\mathrm{g}\right)\:+\:\mathrm{Cl}_{\mathrm{2}} \left(\mathrm{g}\right)\:\rightarrow\:\mathrm{CH}_{\mathrm{3}} \mathrm{Cl}\left(\mathrm{g}\right) \\ $$$$+\:\mathrm{HCl}\left(\mathrm{g}\right)\:\mathrm{has}\:\Delta\mathrm{H}\:=\:−\mathrm{25}\:\mathrm{kcal}\:\mathrm{and}\:\mathrm{bond} \\ $$$$\mathrm{dissociation}\:\mathrm{energy}\:\mathrm{of}\:\mathrm{C}\:−\:\mathrm{Cl},\:\mathrm{H}\:−\:\mathrm{Cl}, \\ $$$$\mathrm{C}\:−\:\mathrm{H}\:\mathrm{and}\:\mathrm{Cl}\:−\:\mathrm{Cl}\:\mathrm{is}\:\mathrm{given}\:\mathrm{as}\:\mathrm{84}\:\mathrm{kcal}/\mathrm{mol}, \\ $$$$\mathrm{103}\:\mathrm{kcal}/\mathrm{mol},\:\mathrm{x}\:\mathrm{kcal}/\mathrm{mol}\:\mathrm{and}\:\mathrm{y}\:\mathrm{kcal}/\mathrm{mol} \\ $$$$\mathrm{respectively}.\:\mathrm{Given}\:\mathrm{x}\::\:\mathrm{y}\:=\:\mathrm{9}\::\:\mathrm{5},\:\mathrm{then} \\ $$$$\mathrm{bond}\:\mathrm{energy}\:\mathrm{of}\:\mathrm{Cl}\:−\:\mathrm{Cl}\:\mathrm{bond}\:\mathrm{in}\:\mathrm{kcal}/\mathrm{mol} \\ $$$$\mathrm{is} \\ $$
Answered by Tinkutara last updated on 31/Oct/17

Commented by Tinkutara last updated on 31/Oct/17

Commented by Tinkutara last updated on 31/Oct/17

