
Question Number 23361 by Tinkutara last updated on 29/Oct/17

$$\mathrm{A}\:\mathrm{50}\:\mathrm{g}\:\mathrm{lead}\:\mathrm{bullet},\:\mathrm{sp}.\:\mathrm{heat}\:\mathrm{0}.\mathrm{02}\:\mathrm{is} \\ $$$$\mathrm{initially}\:\mathrm{at}\:\mathrm{30}°\mathrm{C}.\:\mathrm{It}\:\mathrm{is}\:\mathrm{fired}\:\mathrm{vertically} \\ $$$$\mathrm{upwards}\:\mathrm{with}\:\mathrm{a}\:\mathrm{speed}\:\mathrm{of}\:\mathrm{840}\:\mathrm{m}/\mathrm{s}\:\mathrm{and} \\ $$$$\mathrm{on}\:\mathrm{returning}\:\mathrm{to}\:\mathrm{the}\:\mathrm{starting}\:\mathrm{level}\:\mathrm{strikes} \\ $$$$\mathrm{a}\:\mathrm{cake}\:\mathrm{of}\:\mathrm{ice}\:\mathrm{at}\:\mathrm{0}°\mathrm{C}.\:\mathrm{How}\:\mathrm{much}\:\mathrm{ice}\:\mathrm{is} \\ $$$$\mathrm{melted}?\:\mathrm{Assume}\:\mathrm{that}\:\mathrm{all}\:\mathrm{energy}\:\mathrm{is} \\ $$$$\mathrm{spent}\:\mathrm{in}\:\mathrm{melting}\:\mathrm{only}\:\left({L}\:=\:\mathrm{80}\:\mathrm{cal}/\mathrm{g}\right). \\ $$
Answered by ajfour last updated on 29/Oct/17
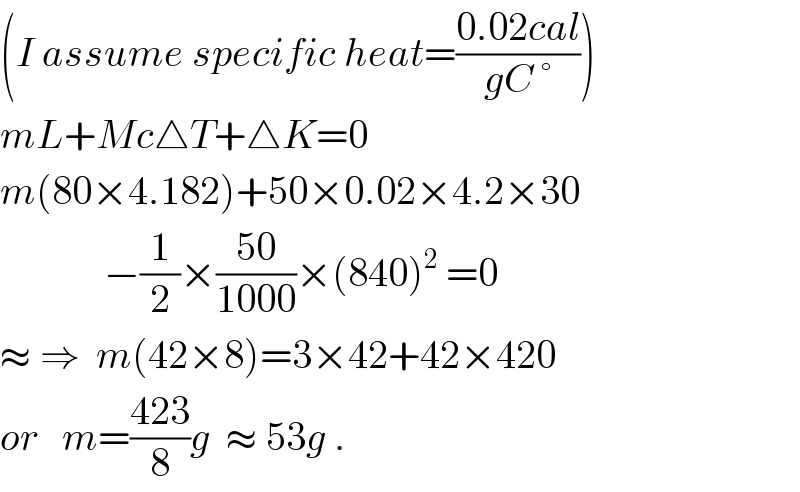
$$\left({I}\:{assume}\:{specific}\:{heat}=\frac{\mathrm{0}.\mathrm{02}{cal}}{{gC}\:°}\right) \\ $$$${mL}+{Mc}\bigtriangleup{T}+\bigtriangleup{K}=\mathrm{0} \\ $$$${m}\left(\mathrm{80}×\mathrm{4}.\mathrm{182}\right)+\mathrm{50}×\mathrm{0}.\mathrm{02}×\mathrm{4}.\mathrm{2}×\mathrm{30} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:−\frac{\mathrm{1}}{\mathrm{2}}×\frac{\mathrm{50}}{\mathrm{1000}}×\left(\mathrm{840}\right)^{\mathrm{2}} \:=\mathrm{0} \\ $$$$\approx\:\Rightarrow\:\:{m}\left(\mathrm{42}×\mathrm{8}\right)=\mathrm{3}×\mathrm{42}+\mathrm{42}×\mathrm{420} \\ $$$${or}\:\:\:{m}=\frac{\mathrm{423}}{\mathrm{8}}{g}\:\:\approx\:\mathrm{53}{g}\:. \\ $$
Commented by Tinkutara last updated on 29/Oct/17

$$\mathrm{Thank}\:\mathrm{you}\:\mathrm{very}\:\mathrm{much}\:\mathrm{Sir}! \\ $$
Commented by ajfour last updated on 29/Oct/17
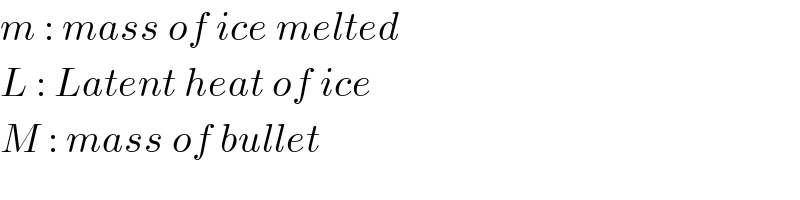
$${m}\::\:{mass}\:{of}\:{ice}\:{melted} \\ $$$${L}\::\:{Latent}\:{heat}\:{of}\:{ice} \\ $$$${M}\::\:{mass}\:{of}\:{bullet} \\ $$
