
Question Number 23694 by Tinkutara last updated on 04/Nov/17
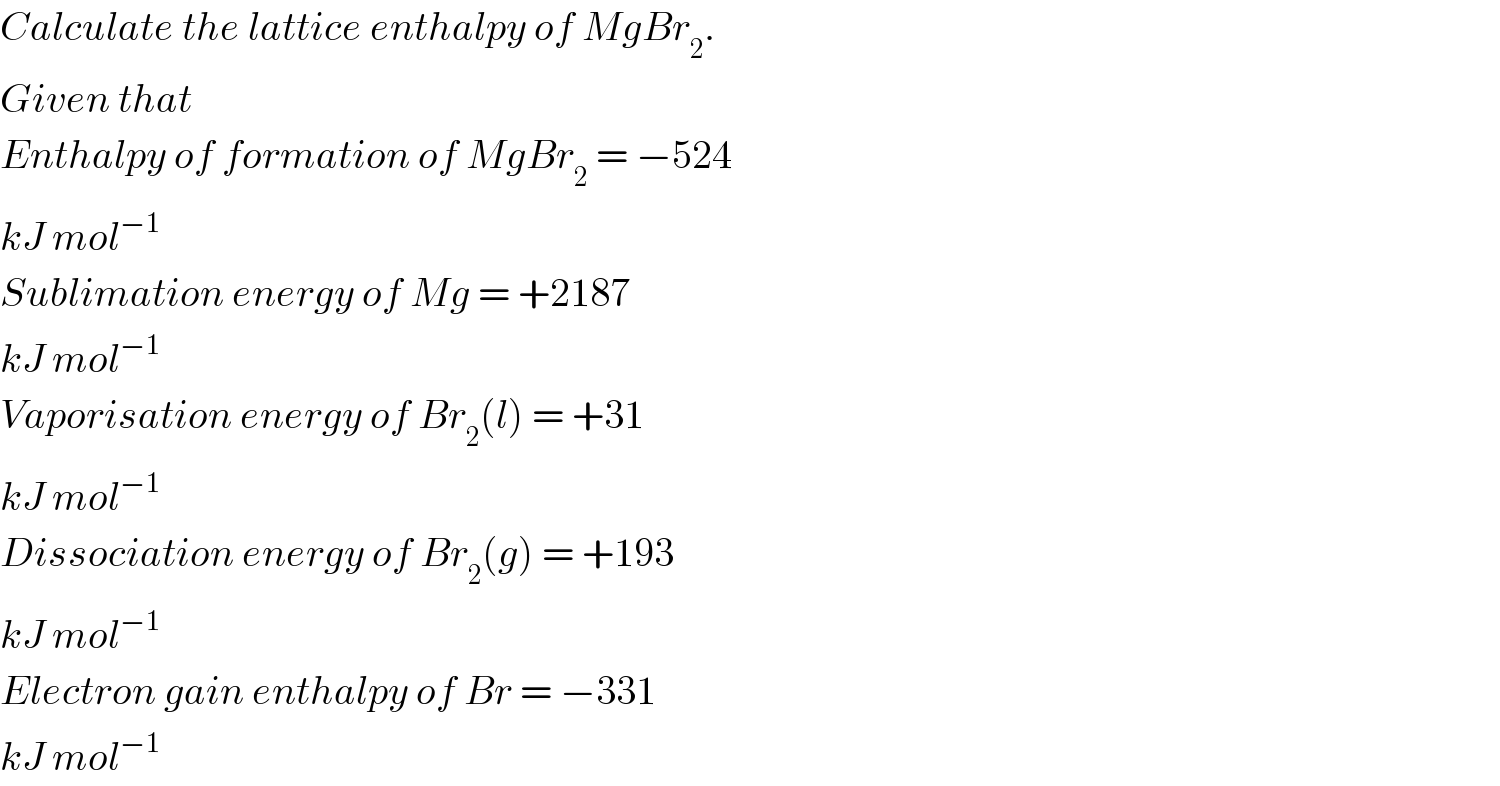
$${Calculate}\:{the}\:{lattice}\:{enthalpy}\:{of}\:{MgBr}_{\mathrm{2}} . \\ $$$${Given}\:{that} \\ $$$${Enthalpy}\:{of}\:{formation}\:{of}\:{MgBr}_{\mathrm{2}} \:=\:−\mathrm{524} \\ $$$${kJ}\:{mol}^{−\mathrm{1}} \\ $$$${Sublimation}\:{energy}\:{of}\:{Mg}\:=\:+\mathrm{2187} \\ $$$${kJ}\:{mol}^{−\mathrm{1}} \\ $$$${Vaporisation}\:{energy}\:{of}\:{Br}_{\mathrm{2}} \left({l}\right)\:=\:+\mathrm{31} \\ $$$${kJ}\:{mol}^{−\mathrm{1}} \\ $$$${Dissociation}\:{energy}\:{of}\:{Br}_{\mathrm{2}} \left({g}\right)\:=\:+\mathrm{193} \\ $$$${kJ}\:{mol}^{−\mathrm{1}} \\ $$$${Electron}\:{gain}\:{enthalpy}\:{of}\:{Br}\:=\:−\mathrm{331} \\ $$$${kJ}\:{mol}^{−\mathrm{1}} \\ $$
