
Question Number 24303 by Tinkutara last updated on 15/Nov/17

$$\boldsymbol{\mathrm{Assertion}}:\:\mathrm{Enthalpy}\:\mathrm{of}\:\mathrm{combustion}\:\mathrm{is} \\ $$$$\mathrm{negative}. \\ $$$$\boldsymbol{\mathrm{Reason}}:\:\mathrm{Combustion}\:\mathrm{reaction}\:\mathrm{can}\:\mathrm{be} \\ $$$$\mathrm{exothermic}\:\mathrm{or}\:\mathrm{endothermic}. \\ $$
Answered by Physics lover last updated on 15/Nov/17

$${assertion}\:{is}\:{right}\:{but}\:{reason}\:{is} \\ $$$${wrong}.{combustion}\:{reaction}\: \\ $$$${is}\:{always}\:{exothermic}. \\ $$
Commented by Tinkutara last updated on 15/Nov/17

$$\mathrm{Book}\:\mathrm{says}\:\mathrm{both}\:\mathrm{correct}\:\mathrm{and}\:\mathrm{reason}\:\mathrm{is} \\ $$$$\mathrm{correct}\:\mathrm{explanation}. \\ $$
Commented by Physics lover last updated on 15/Nov/17

$${hmmm}.{i}\:{will}\:{ask}\:{my}\:{teacher}. \\ $$$${But}\:{i}\:{dont}\:{think}\:{that}\:{can}\:{be}\:{true}. \\ $$
Commented by Physics lover last updated on 15/Nov/17
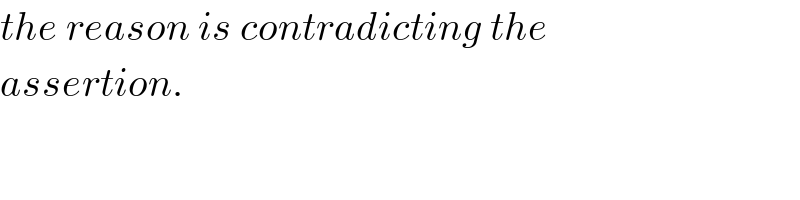
$${the}\:{reason}\:{is}\:{contradicting}\:{the}\: \\ $$$${assertion}. \\ $$
