
Question and Answers Forum
Question Number 28076 by Tinkutara last updated on 20/Jan/18
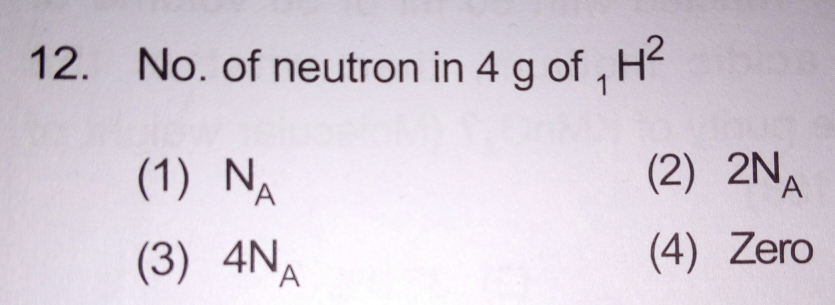
Commented by Tinkutara last updated on 20/Jan/18
Answer given is 3rd. Is the answer correct?
Answered by ajfour last updated on 20/Jan/18
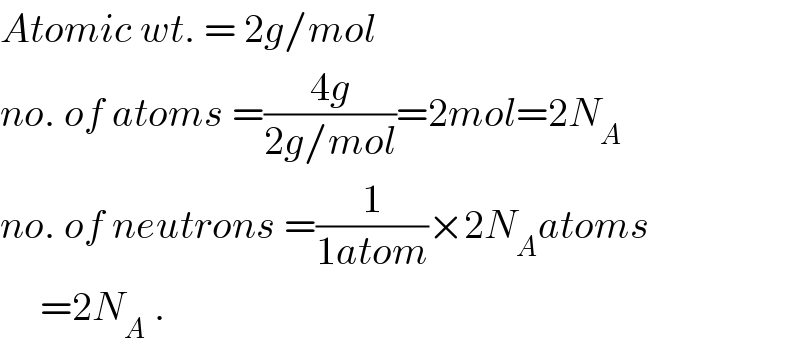
Commented by Tinkutara last updated on 20/Jan/18
Yes this is the same I got thanks but I wanted to confirm the answer.
