
Question and Answers Forum
Question Number 30427 by rahul 19 last updated on 22/Feb/18
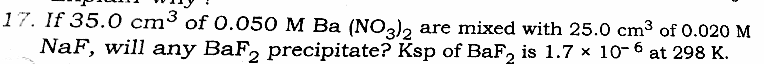
Commented by Tinkutara last updated on 22/Feb/18
Is the answer yes?
Commented by rahul 19 last updated on 22/Feb/18

Commented by Tinkutara last updated on 22/Feb/18
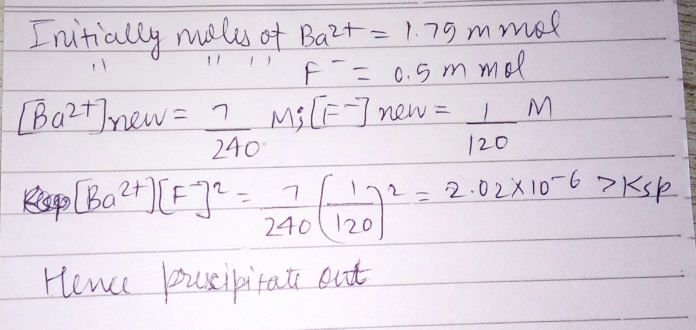
Commented by Tinkutara last updated on 23/Feb/18
![[Ba^(2+) ]=((1.75 m mol)/(60 mL))=(7/(240))M ∵ New volume=25+35=60 mL We do now [Ba^(2+) ][F^− ]^2 to calculate the solubility product of BaF_2 .](Q30536.png)
Commented by rahul 19 last updated on 22/Feb/18
![how [Ba^(2+) ]= 7/240? and also why [F^− ]^(2 ) , acc. to balanced eq. it should be [F^− ].](Q30465.png)
Commented by rahul 19 last updated on 23/Feb/18

