
Question and Answers Forum
Question Number 31619 by Tinkutara last updated on 11/Mar/18

Answered by Joel578 last updated on 11/Mar/18
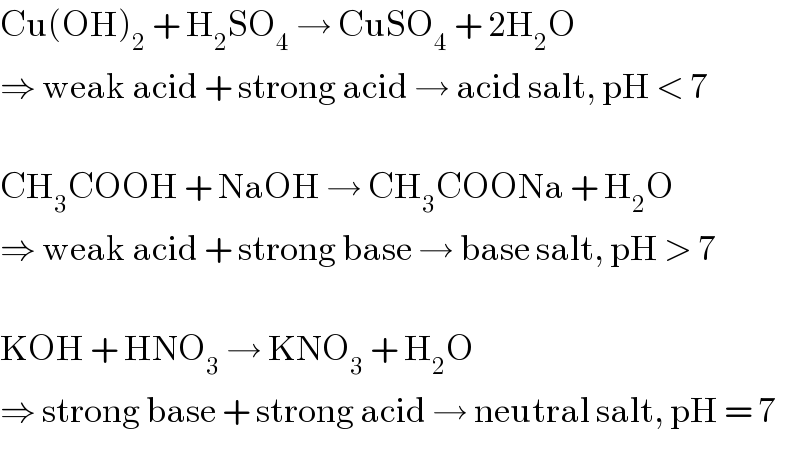
Commented by Tinkutara last updated on 11/Mar/18
Is it a typological error in B column option c?
Commented by Joel578 last updated on 11/Mar/18

Commented by Tinkutara last updated on 11/Mar/18
Thanks Sir! ����☺��
