
Question and Answers Forum
Question Number 31626 by Tinkutara last updated on 11/Mar/18

Answered by Joel578 last updated on 11/Mar/18
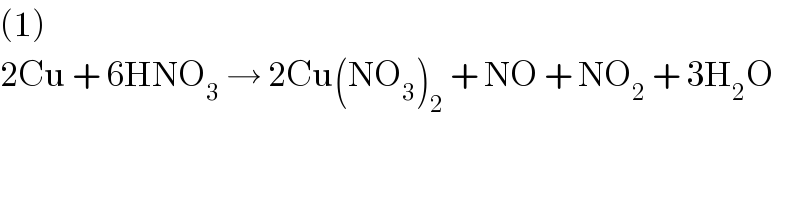
Commented by Tinkutara last updated on 11/Mar/18
Yes I too balanced and got (1). But answer given is 2⃣. That probably came by multiplying the whole equation by 2.
Commented by Joel578 last updated on 11/Mar/18
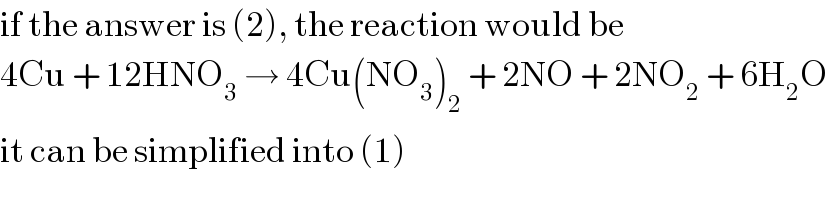
Commented by Joel578 last updated on 12/Mar/18

Commented by Tinkutara last updated on 12/Mar/18
��
