
Question and Answers Forum
Question Number 31906 by Tinkutara last updated on 16/Mar/18

Answered by rahul 19 last updated on 18/Mar/18
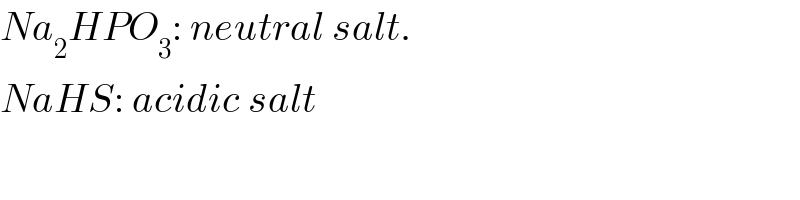
Commented by Tinkutara last updated on 19/Mar/18
Why NaHS=acidic salt? Na is strong base and H2S is weak acid so why not basic salt?
Commented by rahul 19 last updated on 19/Mar/18
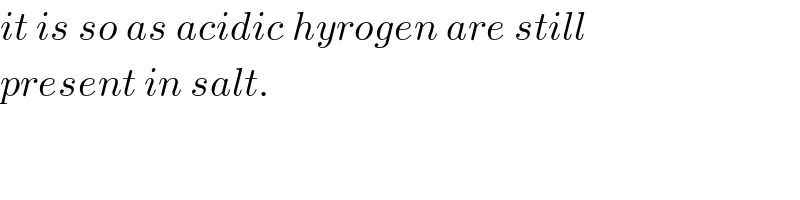
Commented by Tinkutara last updated on 19/Mar/18
But acidic salt means its pH<7 but due to strong base Na it's pH>7. Isn't it wrong then?
Commented by rahul 19 last updated on 19/Mar/18

Commented by Tinkutara last updated on 19/Mar/18
Thank you very much Sir! I got the answer. ��������
