
Question and Answers Forum
Previous in Properties of Matter Next in Properties of Matter
Question Number 34762 by Tinkutara last updated on 10/May/18

Answered by ajfour last updated on 10/May/18

Commented by Tinkutara last updated on 11/May/18

Commented by ajfour last updated on 11/May/18

Commented by Tinkutara last updated on 11/May/18
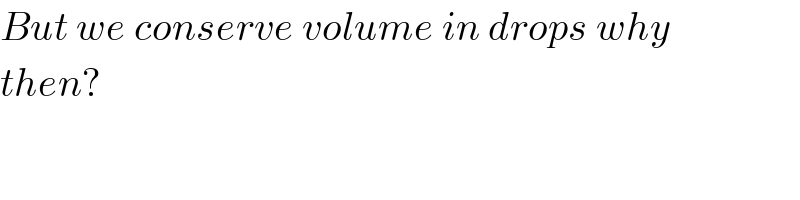
Commented by ajfour last updated on 11/May/18
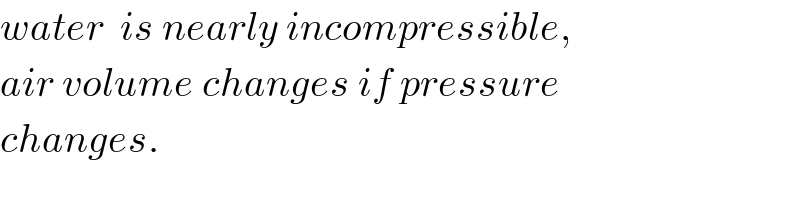
Commented by Tinkutara last updated on 11/May/18
Thank you very much Sir! I got the answer. ��������
