Question and Answers Forum
Previous in Heat and Theromdynamics Next in Heat and Theromdynamics
Question Number 42261 by Necxx last updated on 21/Aug/18
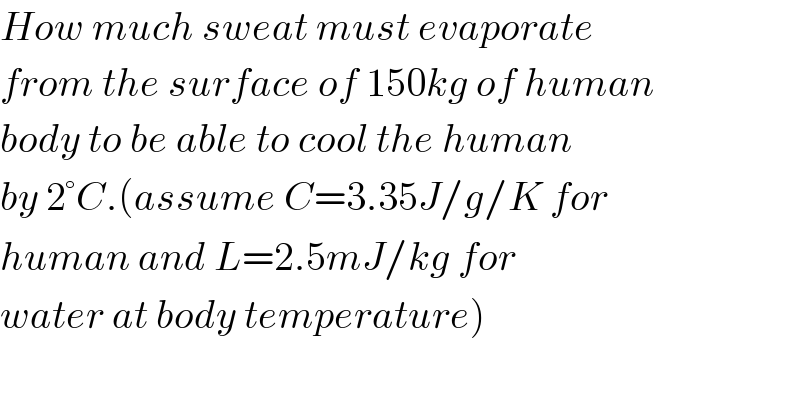
Commented by Necxx last updated on 21/Aug/18

Answered by tanmay.chaudhury50@gmail.com last updated on 21/Aug/18
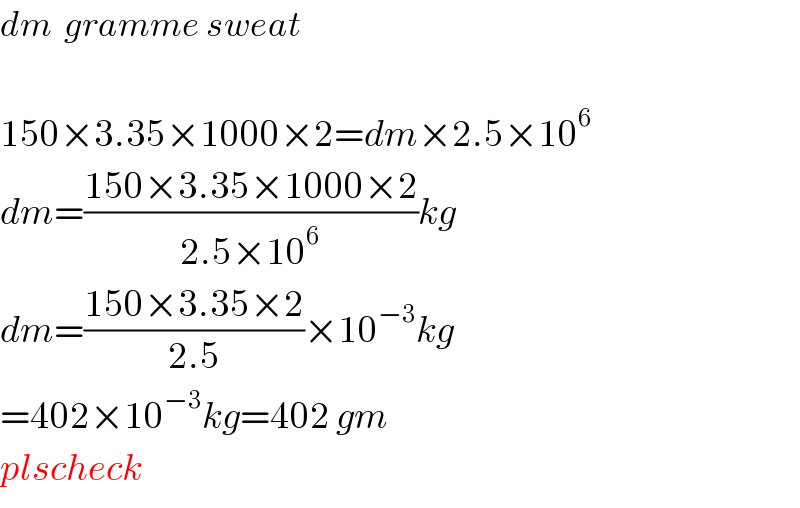
Commented by Necxx last updated on 21/Aug/18