
Previous in Heat and Theromdynamics Next in Heat and Theromdynamics
Question Number 47314 by rahul 19 last updated on 08/Nov/18

$${During}\:{random}\:{motion},\:{gas}\:{molecules} \\ $$$${do}\:{not}\:{interact}\:{with}\:{each}\:{other}\:. \\ $$$${Hence}\:\:{Potential}\:{energy}\:=\mathrm{0}\:...... \\ $$$${Pls}\:{explain}\:{why}\:{P}.{E}\:=\:\mathrm{0}? \\ $$$${P}.{E}\:{is}\:{stored}\:{form}\:{of}\:{energy},{right}? \\ $$
Commented by rahul 19 last updated on 08/Nov/18

$${this}\:{is}\:{one}\:{of}\:{the}\:\:{postulate}\:{of}\:{K}.{T}.{G}.. \\ $$
Answered by ajfour last updated on 08/Nov/18
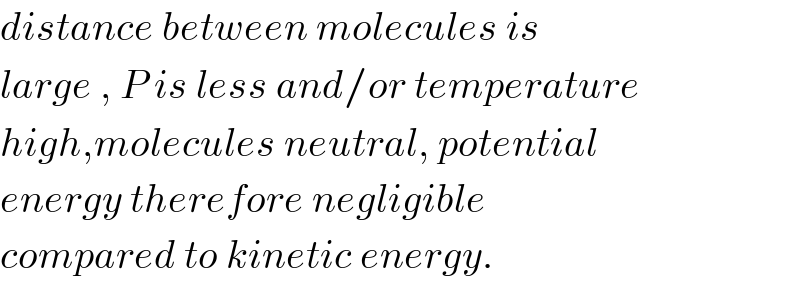
$${distance}\:{between}\:{molecules}\:{is} \\ $$$${large}\:,\:{P}\:{is}\:{less}\:{and}/{or}\:{temperature} \\ $$$${high},{molecules}\:{neutral},\:{potential}\: \\ $$$${energy}\:{therefore}\:{negligible} \\ $$$${compared}\:{to}\:{kinetic}\:{energy}. \\ $$
Commented by rahul 19 last updated on 08/Nov/18

$${Sir},\:{what}'{s}\:{diff}.\:{between} \\ $$$${gas}\:{molecules}\:{interact}\:{with}\:{each}\:{other}. \\ $$$$\&\:{gas}\:{molecules}\:{collide}\:{with}\:{each}\:{other}. \\ $$
Commented by ajfour last updated on 08/Nov/18

$${during}\:{collision},\:{this}\:{is}\:{even}\:{at} \\ $$$${some}\:{distance}\:\left({when}\:{charged}\:{bodies}\right. \\ $$$$\left.{concerned}\right),\:{potential}\:{energy}\:{rises} \\ $$$${considerably}\:{but}\:{after}\:{collision} \\ $$$${returns}\:{back}\:{to}\:{neligible}\:{value} \\ $$$${for}\:{ideal}\:{gas}\:{atoms}.\:{So}\:{as}\:{far}\:{as} \\ $$$${electrostatic}\:{interaction}\:{is}\: \\ $$$${concerned},\:{this}\:{takes}\:{place}\:{only} \\ $$$${during}\:{non}-{contact}\:{collisions}; \\ $$$${contact}\:{collisions}\:{may}\:{result}\:{in} \\ $$$${a}\:{chemical}\:{reaction}. \\ $$
Commented by rahul 19 last updated on 08/Nov/18
thanks sir ����
