
Question and Answers Forum
Question Number 51245 by Tawa1 last updated on 25/Dec/18

Answered by afachri last updated on 25/Dec/18

Commented by Tawa1 last updated on 25/Dec/18

Answered by afachri last updated on 25/Dec/18
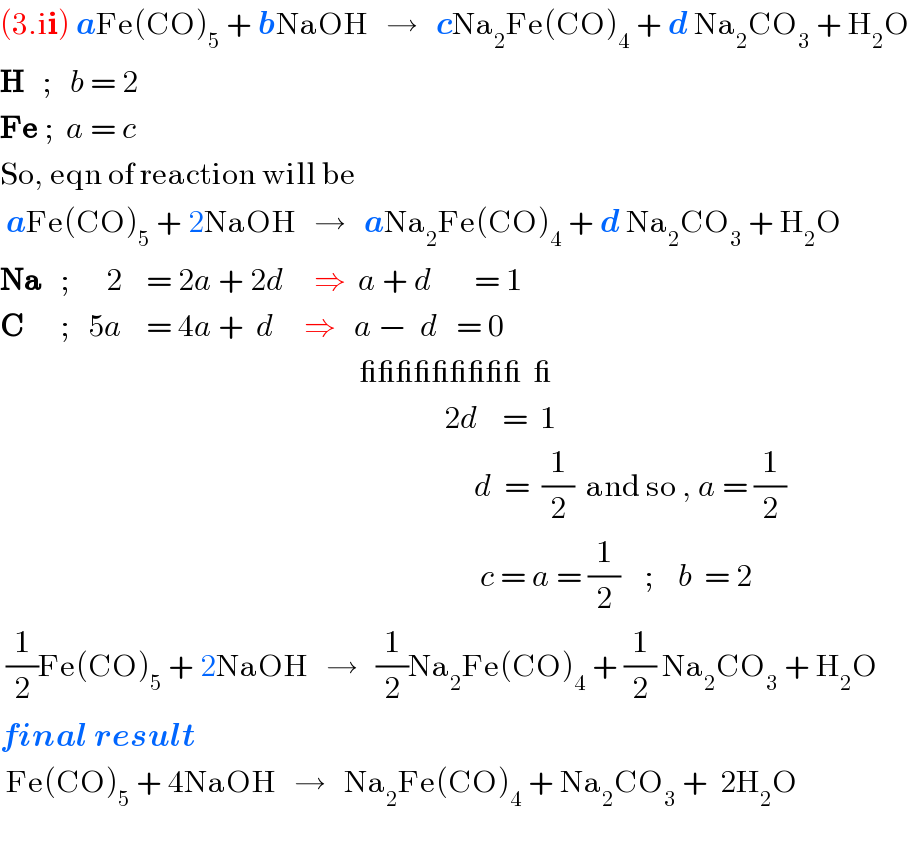
Commented by Tawa1 last updated on 25/Dec/18
Commented by afachri last updated on 25/Dec/18

Commented by rahul 19 last updated on 25/Dec/18
Commented by malwaan last updated on 25/Dec/18
Commented by afachri last updated on 25/Dec/18
Commented by afachri last updated on 25/Dec/18

