
Question and Answers Forum
Question Number 52991 by prakash jain last updated on 16/Jan/19

Commented by prakash jain last updated on 16/Jan/19
Correct option is 2. NTA official key has 3. Please feedback. This question is also from 9th Jan JEE main
Answered by tanmay.chaudhury50@gmail.com last updated on 16/Jan/19
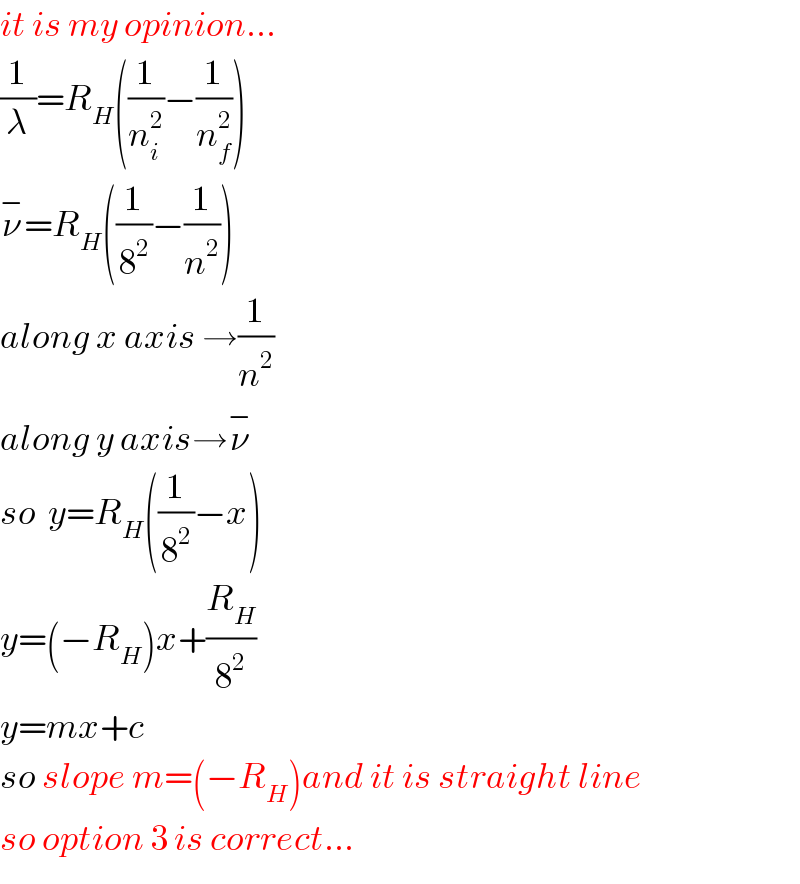
Commented by prakash jain last updated on 16/Jan/19
![(1/λ)=R[(1/n_f ^2 )−(1/n_i ^2 )] I did a google search.](Q53003.png)
Commented by prakash jain last updated on 16/Jan/19
Commented by prakash jain last updated on 16/Jan/19

Commented by prakash jain last updated on 16/Jan/19
Also the question says 8 to n. for example Balmer series emission spectrum is (1/2^2 - 1/n^2). From n to 2. So from 8 to n will mean n has to smaller than 8 for it to be emission spectrum.
Commented by tanmay.chaudhury50@gmail.com last updated on 16/Jan/19
Commented by tanmay.chaudhury50@gmail.com last updated on 16/Jan/19

Commented by tanmay.chaudhury50@gmail.com last updated on 16/Jan/19

Commented by tanmay.chaudhury50@gmail.com last updated on 16/Jan/19

Commented by tanmay.chaudhury50@gmail.com last updated on 16/Jan/19

Commented by prakash jain last updated on 16/Jan/19

Commented by prakash jain last updated on 16/Jan/19

Commented by prakash jain last updated on 16/Jan/19

Commented by tanmay.chaudhury50@gmail.com last updated on 16/Jan/19

Commented by tanmay.chaudhury50@gmail.com last updated on 16/Jan/19

