
Question and Answers Forum
Question Number 55308 by Tawa1 last updated on 21/Feb/19

Answered by Rio Mike last updated on 21/Feb/19
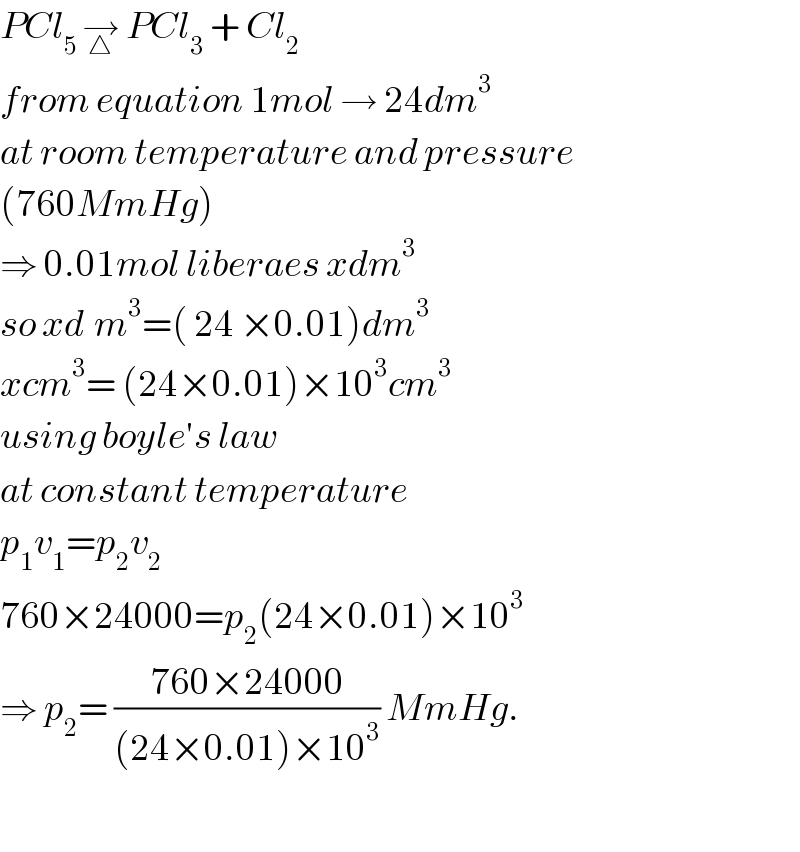
Commented by Tawa1 last updated on 23/Feb/19
| ||
Question and Answers Forum | ||
Question Number 55308 by Tawa1 last updated on 21/Feb/19 | ||
 | ||
Answered by Rio Mike last updated on 21/Feb/19 | ||
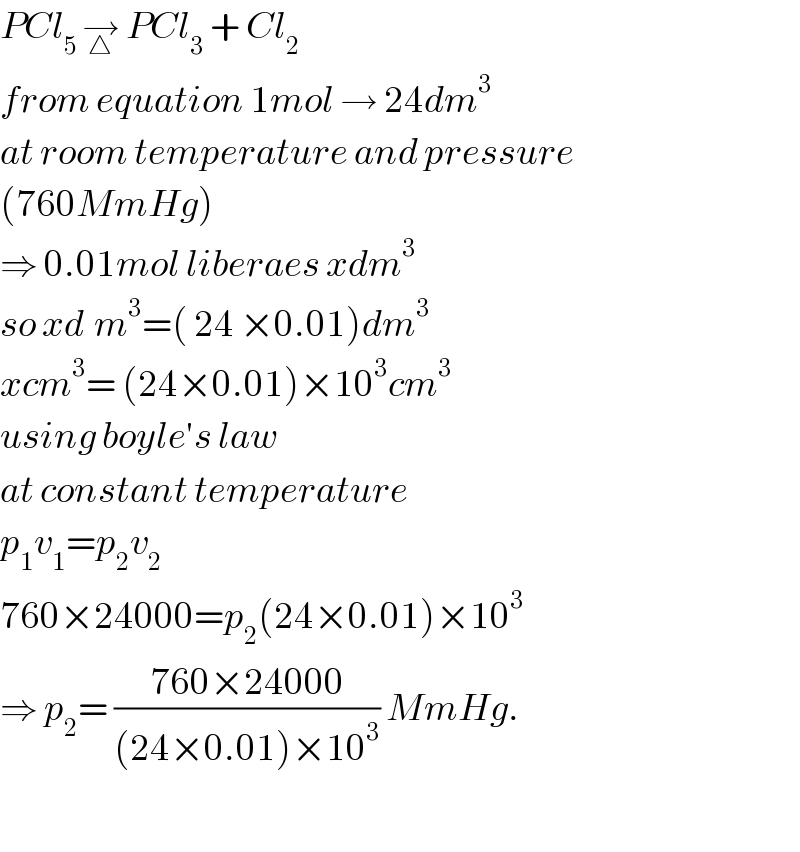 | ||
| ||
Commented by Tawa1 last updated on 23/Feb/19 | ||
 | ||