
Question and Answers Forum
Question Number 57785 by Tinkutara last updated on 11/Apr/19

Commented by rahul 19 last updated on 14/Apr/19

Commented by Tinkutara last updated on 13/Apr/19
How?
Answered by rahul 19 last updated on 13/Apr/19

Commented by rahul 19 last updated on 16/Apr/19
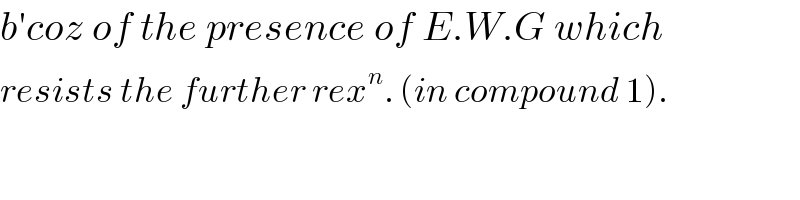
Commented by Tinkutara last updated on 14/Apr/19
Why the second product is chosen not first?
Commented by Tinkutara last updated on 16/Apr/19
Ok but why O of benzene ring not get bonded to AlCl3 initially?
Commented by rahul 19 last updated on 16/Apr/19
1)it will lead to the same compound you started with. at the end Oh will be form . 2) show me your final product what exactly you want to do.
Commented by Tinkutara last updated on 18/Apr/19
How do you removed OH on the carbonyl carbon? Please check whether I am correct:
Commented by Tinkutara last updated on 18/Apr/19

Commented by rahul 19 last updated on 18/Apr/19
read my 1st comment.... I'm saying that C=O grp is electron withdrawing grp. → deactivates the ring → E.A.S not possible or we can say it will never be the major product as asked in the Question.
Commented by Tinkutara last updated on 18/Apr/19
Ohh yes, and how did you remove the OH group on the carbonyl carbon on the product with ✔ ?
Commented by rahul 19 last updated on 18/Apr/19
AlCl3 which is regenerated will remove OH .��
Commented by Tinkutara last updated on 19/Apr/19
Thanks a lot!☺��
