
Question and Answers Forum
Previous in Heat and Theromdynamics Next in Heat and Theromdynamics
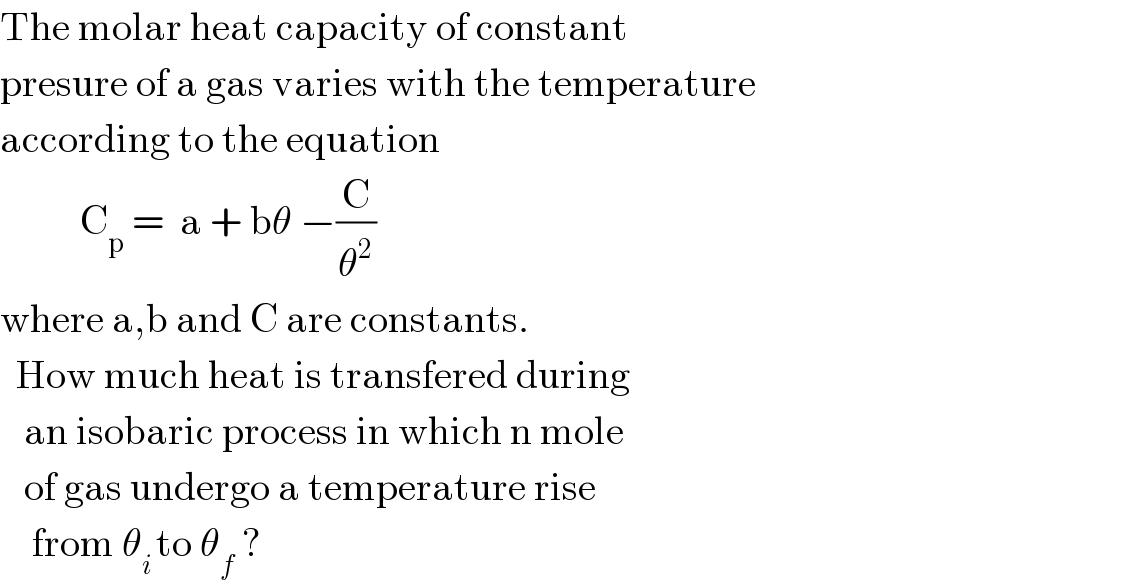
Question Number 58196 by Umar last updated on 19/Apr/19
Answered by tanmay last updated on 19/Apr/19
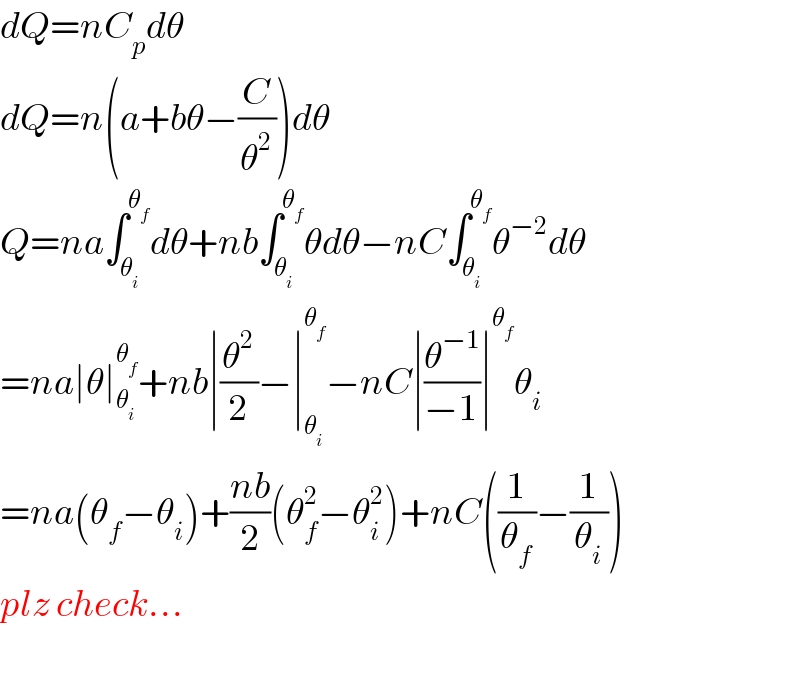
Commented by Umar last updated on 19/Apr/19

| ||
Question and Answers Forum | ||
Previous in Heat and Theromdynamics Next in Heat and Theromdynamics | ||
Question Number 58196 by Umar last updated on 19/Apr/19 | ||
 | ||
Answered by tanmay last updated on 19/Apr/19 | ||
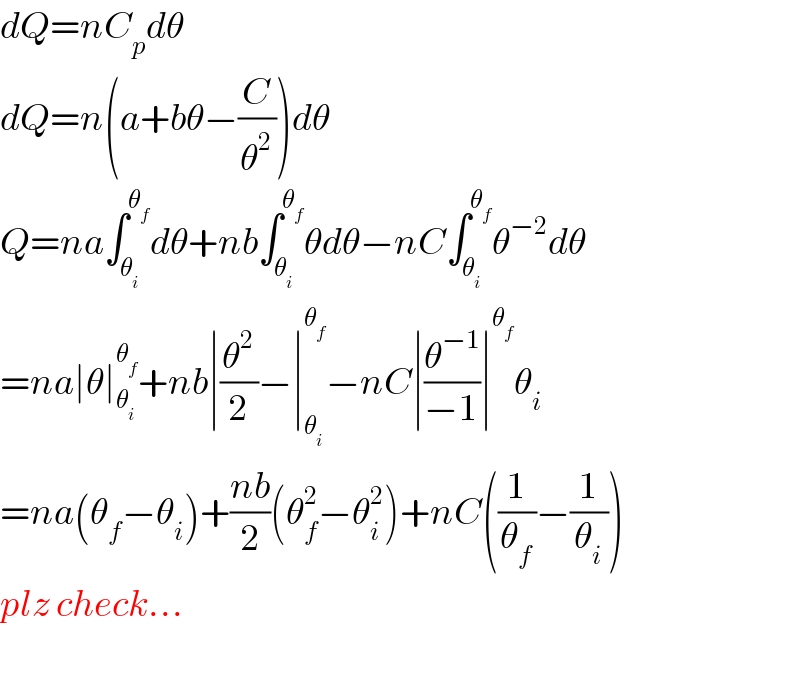 | ||
| ||
Commented by Umar last updated on 19/Apr/19 | ||
 | ||