
Question and Answers Forum
Question Number 62923 by peter frank last updated on 26/Jun/19

Answered by peter frank last updated on 27/Jun/19
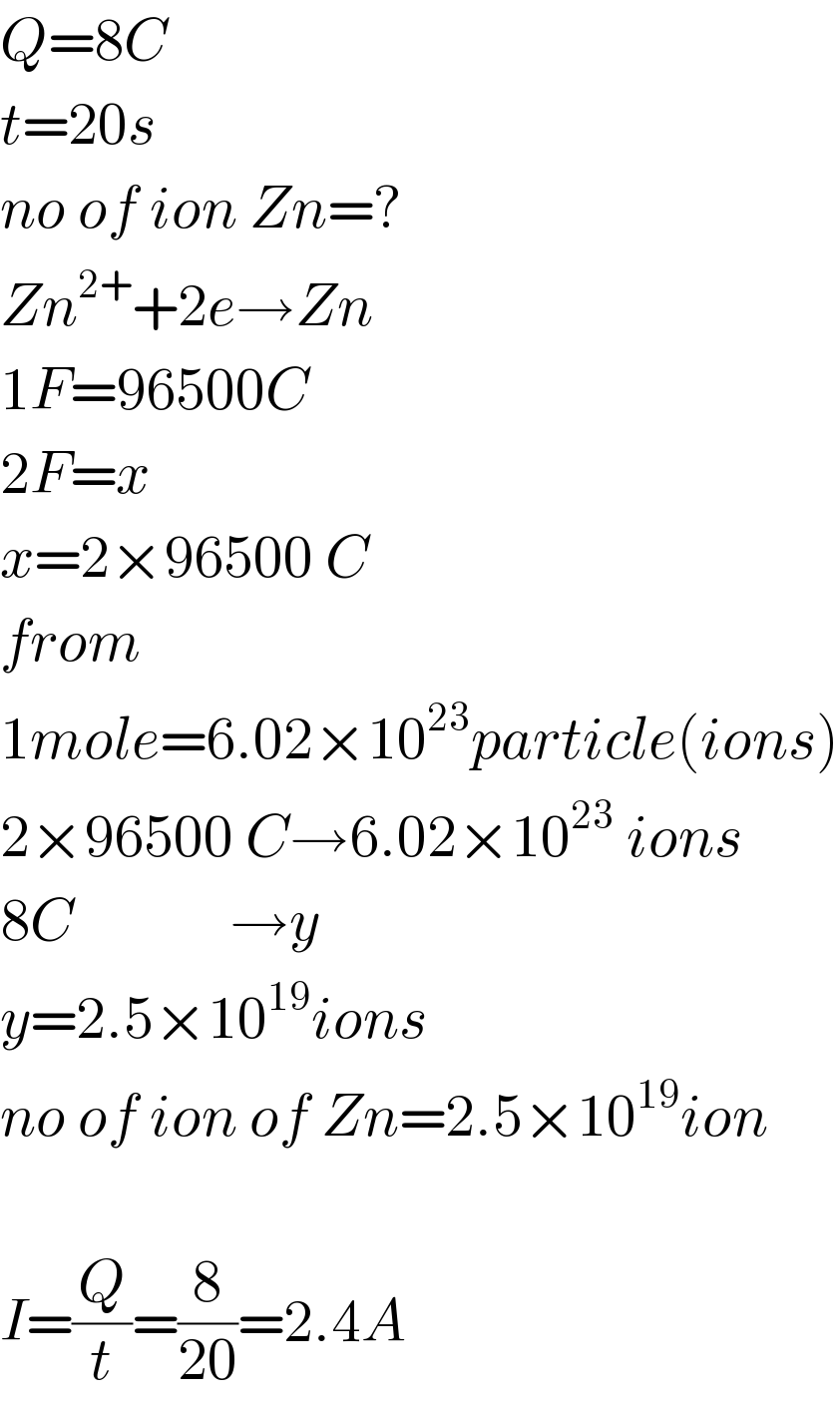
Answered by peter frank last updated on 27/Jun/19

| ||
Question and Answers Forum | ||
Question Number 62923 by peter frank last updated on 26/Jun/19 | ||
 | ||
Answered by peter frank last updated on 27/Jun/19 | ||
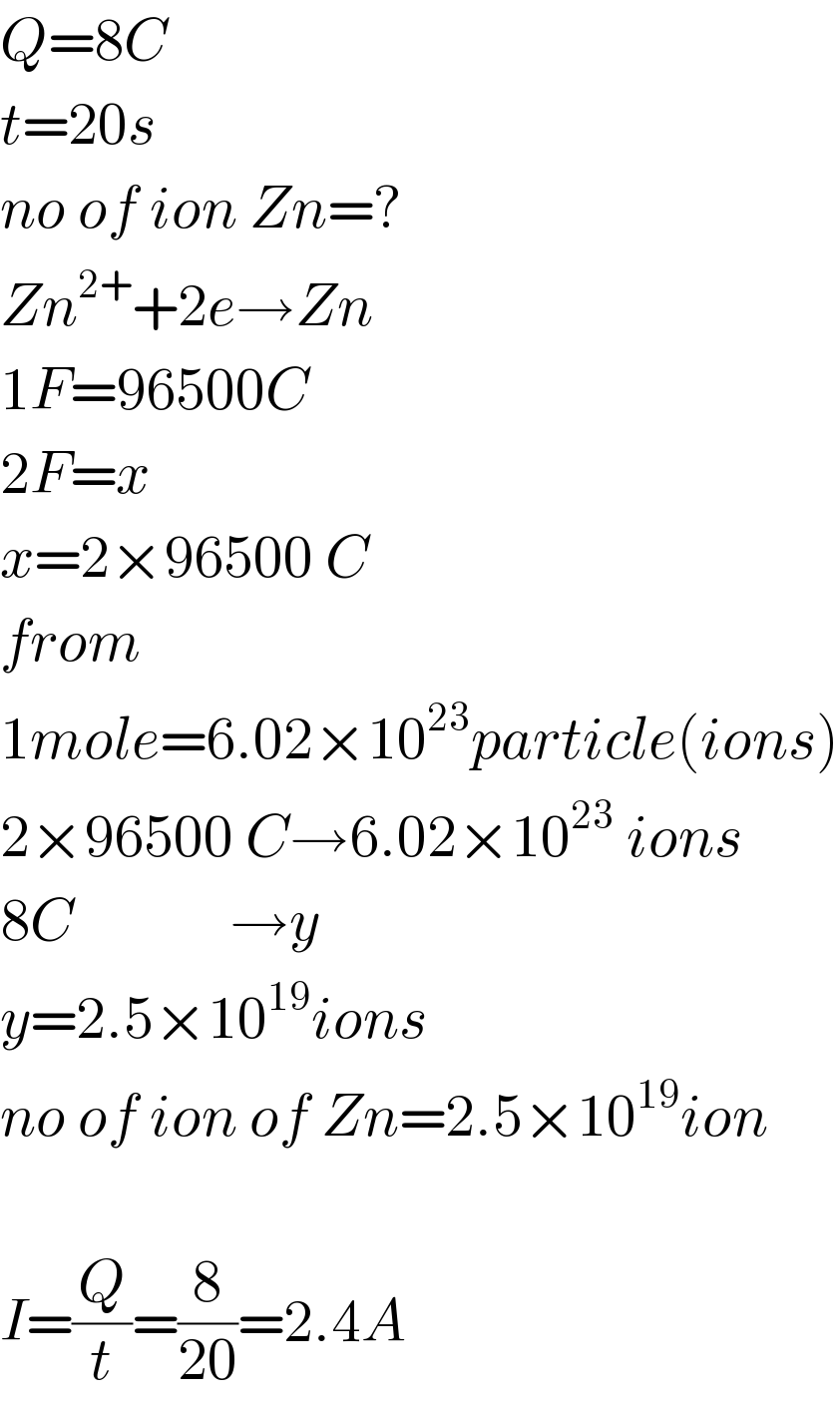 | ||
| ||
Answered by peter frank last updated on 27/Jun/19 | ||
 | ||
| ||