
Question Number 6583 by Tawakalitu. last updated on 04/Jul/16

$${An}\:{aqeous}\:{solution}\:{of}\:{tetraoxosulphate}\left({IV}\right)\:{has}\:{a}\:{density}\: \\ $$$${of}\:\mathrm{1}.\mathrm{80}\:{g}/{cm}^{\mathrm{3}} \:{and}\:\mathrm{98\%}\:{purity}\:{level}.\:{what}\:{volume}\:{of}\:{this}\: \\ $$$${solution}\:{must}\:{be}\:{diluted}\:{to}\:{give}\:\mathrm{250}\:{cm}^{\mathrm{3}} \:{of}\:\mathrm{0}.\mathrm{500}\:{mol}/{dm}^{\mathrm{3}} \\ $$$${H}_{\mathrm{2}} {SO}_{\mathrm{4}} \:{solution}\:? \\ $$$$ \\ $$
Answered by sandy_suhendra last updated on 04/Jul/16
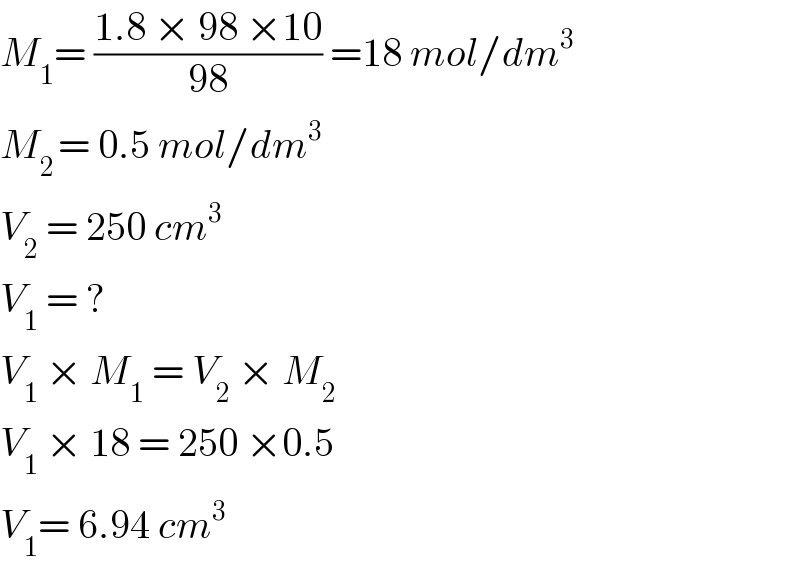
$${M}_{\mathrm{1}} =\:\frac{\mathrm{1}.\mathrm{8}\:×\:\mathrm{98}\:×\mathrm{10}}{\mathrm{98}}\:=\mathrm{18}\:{mol}/{dm}^{\mathrm{3}} \\ $$$${M}_{\mathrm{2}\:} =\:\mathrm{0}.\mathrm{5}\:{mol}/{dm}^{\mathrm{3}} \\ $$$${V}_{\mathrm{2}} \:=\:\mathrm{250}\:{cm}^{\mathrm{3}} \\ $$$${V}_{\mathrm{1}} \:=\:? \\ $$$${V}_{\mathrm{1}} \:×\:{M}_{\mathrm{1}} \:=\:{V}_{\mathrm{2}} \:×\:{M}_{\mathrm{2}} \\ $$$${V}_{\mathrm{1}} \:×\:\mathrm{18}\:=\:\mathrm{250}\:×\mathrm{0}.\mathrm{5} \\ $$$${V}_{\mathrm{1}} =\:\mathrm{6}.\mathrm{94}\:{cm}^{\mathrm{3}} \\ $$
Commented by sandy_suhendra last updated on 04/Jul/16
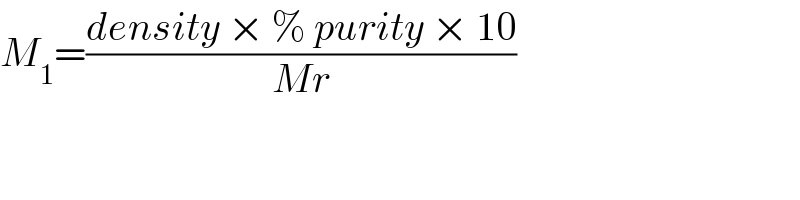
$${M}_{\mathrm{1}} =\frac{{density}\:×\:\%\:{purity}\:×\:\mathrm{10}}{{Mr}} \\ $$
Commented by Tawakalitu. last updated on 05/Jul/16

$${Thanks}\:{so}\:{much} \\ $$
