
Question and Answers Forum
Question Number 75860 by peter frank last updated on 18/Dec/19
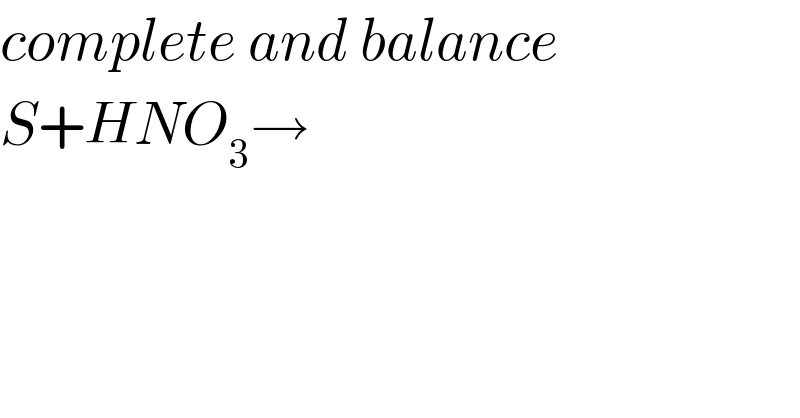
Answered by Crabby89p13 last updated on 19/Dec/19
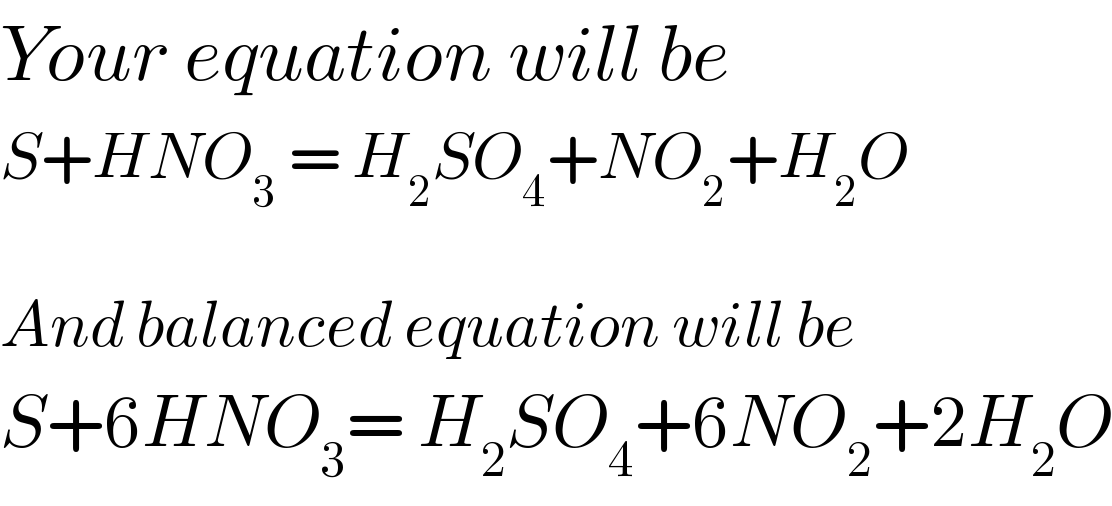
Commented by peter frank last updated on 19/Dec/19

| ||
Question and Answers Forum | ||
Question Number 75860 by peter frank last updated on 18/Dec/19 | ||
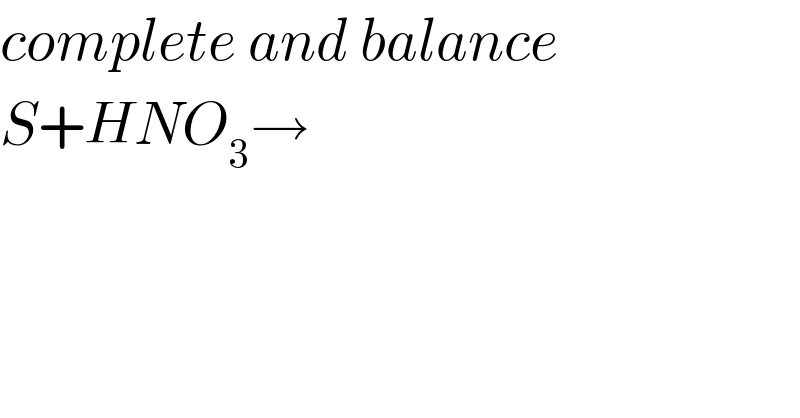 | ||
Answered by Crabby89p13 last updated on 19/Dec/19 | ||
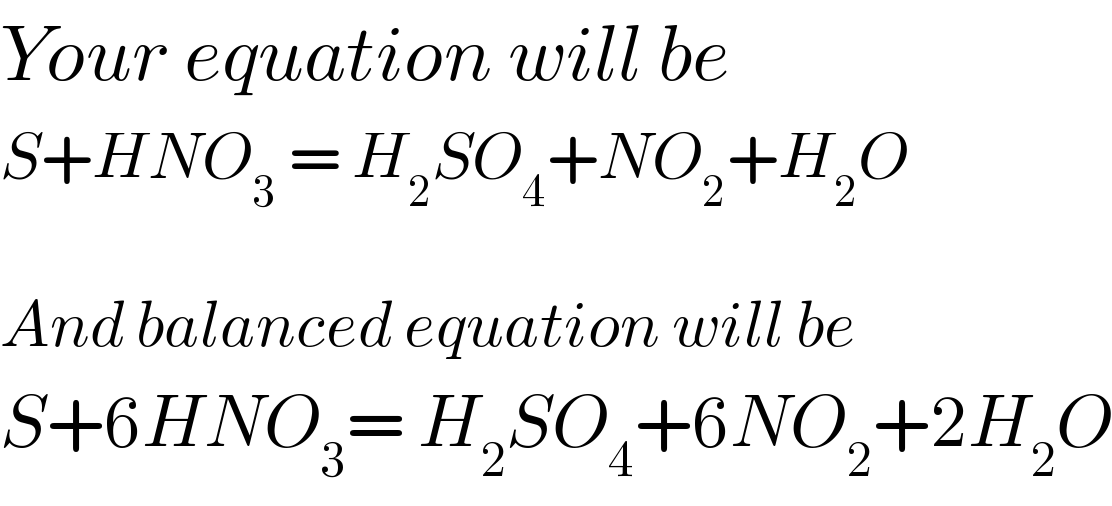 | ||
| ||
Commented by peter frank last updated on 19/Dec/19 | ||
 | ||