
Question and Answers Forum
Atomic StructureQuestion and Answers: Page 1


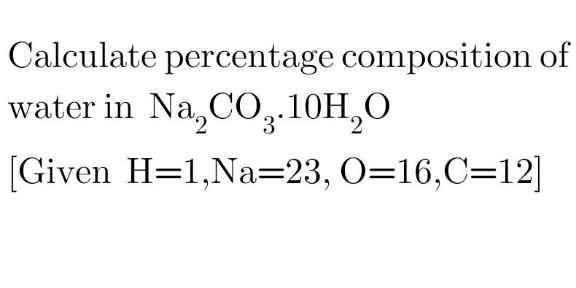







|
Question and Answers Forum |
Atomic StructureQuestion and Answers: Page 1 |

|
| Write a balanced Ionic chemical equation for the following reaction (a)HCl+CaCO_3 →CaCl_2 +CO_(2(g)) +H_2 O_((l)) (b)NH_4 OH_( (aq)) +HCl_((aq)) →NH_4 Cl_((aq)) +H_2 O_((l)) 31/1/2024 |
| in the malicol of CaCO_3 how many σ and π bond has? |
| how is solution 72.5gr of the [C_3 H_6 O] how many Molecule of [H] exist? |
| how is solution 72.5gr of the [C_3 H_6 O] how many Molecule of [H] exist? |
| Give the IUPAC name of the following (a)H_3 PO_4 (b)H_2 CO_3 (c)HCIO (d) HNO_2 (e)HNO_3 (f)Al_2 (SO_4 )_3 (g)Ca_3 (PO_4 )_2 (h)MgO_2 (i)HF |
| 8.1g of a compound Q contain magnessium and oxygen.if mass of magnessium is 4.9g Determine the empirical formular of compound Q. [given Mg=24 O=16] |
| Using Electronic diagrams show the bonding in the following (a)Magnesium chloride (b)Sodium chloride |
| Hydrocarbon contain 80% by mass of carbon and the rest is for hydrogen.calculate the empirical formula of the compound [given H=1 C=12] |

|
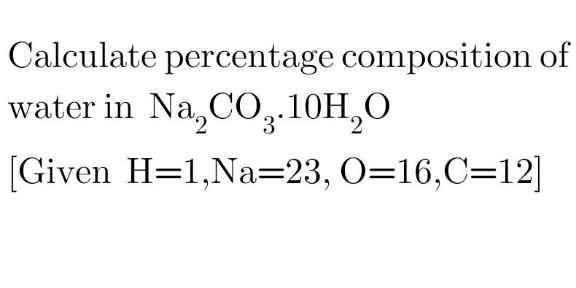
|

|
| 4+x+2x=8+2(3+x)−3 Find x |
| Work out and sketch P_y orbitals |
| work out and sketch d−orbital for the function i) f_1 =3Cos^2 θ−1 i) f_2 =SinθCosθCosφ |

|

|

|

|

|
| complete and balance S+HNO_3 → |
| why the first ionisation(△H_(i1) )energy of Oxygen smaller than the second? A. Due to the nuclear charge B. Due to the distance of the electron from the nucleus C. Due to the effect of spin−pair repulsion D. Due to a shielding effect. |
| JEE Main chemistry errors by Resonance. Today is the last day for raising objectiions. You need to tap once to select and tap the text again to open hyperlink. |

|
| how can i derive a formula to calculate the speed of an electron in n^(th) orbit of a hydrogen atom? |

|