
Question and Answers Forum
Chemical KineticsQuestion and Answers: Page 1
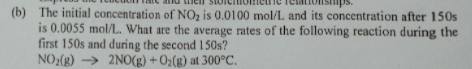

|
Question and Answers Forum |
Chemical KineticsQuestion and Answers: Page 1 |
| NO_2 (g) ⇒ 2NO (g)+O_2 (g) at 300°C The initial concentration of NO_2 is 0.01 mol/L and its concentration after 150 s is .0055 mol/L. What are the average rates of the above reaction during the first 150 s and during the second 150 s? |
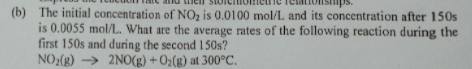
|

|