Question and Answers Forum
Question Number 139619 by I want to learn more last updated on 29/Apr/21

Answered by mr W last updated on 30/Apr/21
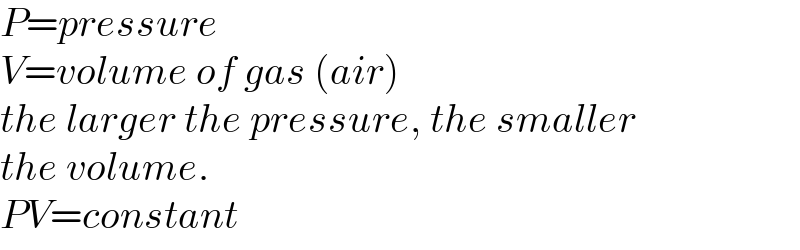
Commented by mr W last updated on 30/Apr/21

Commented by mr W last updated on 30/Apr/21

Commented by I want to learn more last updated on 30/Apr/21