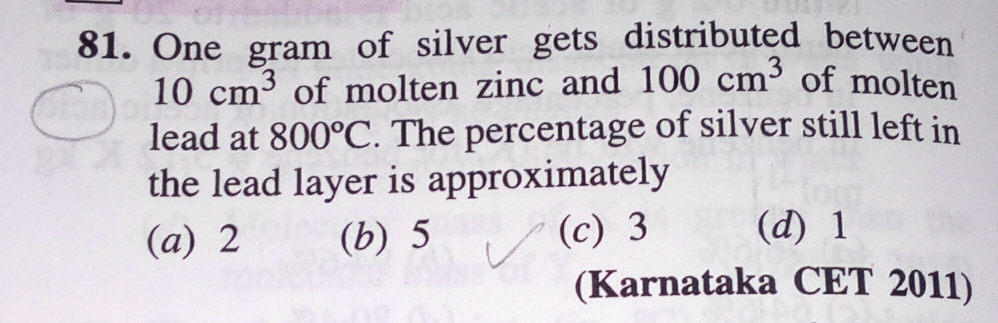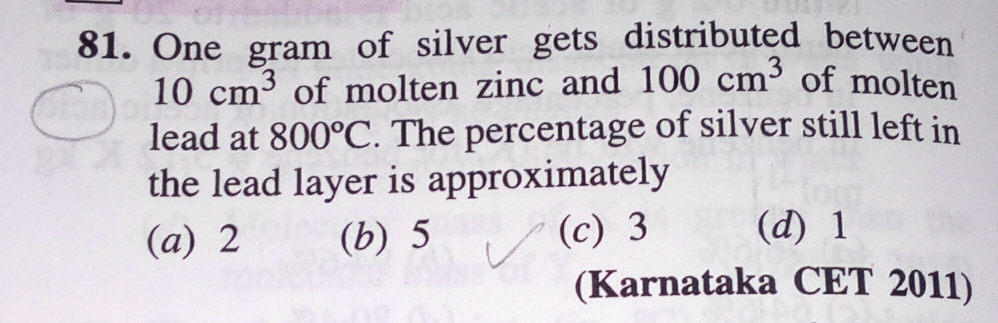|
|

|
All Questions Topic List |
SolutionsQuestion and Answers: Page 1 |
Question Number 189797 Answers: 0 Comments: 2
|

|
Question Number 178166 Answers: 1 Comments: 0
|
| 8.5g of hydrated copper (ii)
sulphate CuSO_4 .xH_2 O was heated
to dryness.if 4.0g of anhydrous
copper (ii) sulphate[CuSO_4 ] obtain
Find number of molecule of water of
crystallization
|
|
Question Number 178165 Answers: 2 Comments: 0
|
| 2.86g of hydrated Sodium carbonate
Na_2 CO_3 .nH_2 O was dissolved
into water to make 250cm^3 solution
of 0.04M Find the value of n
|
|
Question Number 152971 Answers: 0 Comments: 0
|

|
Question Number 136205 Answers: 0 Comments: 0
|

|
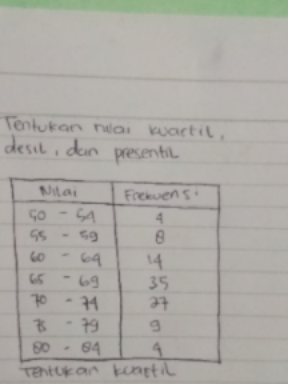
Question Number 112848 Answers: 0 Comments: 1
|
|
Question Number 69159 Answers: 0 Comments: 0
|
| please help
A 7.38 g sample of magnesium sulfate MgSO_4 . X H_2 O lost 3.78g of
water on heating, find the value of X.
|
|
Question Number 64078 Answers: 0 Comments: 3
|

|
Question Number 64077 Answers: 0 Comments: 0
|

|
Question Number 62923 Answers: 2 Comments: 0
|

|
Question Number 62885 Answers: 0 Comments: 0
|

|
Question Number 52708 Answers: 0 Comments: 0
|

|
Question Number 52299 Answers: 0 Comments: 0
|

|
Question Number 52139 Answers: 0 Comments: 0
|

|
Question Number 41369 Answers: 0 Comments: 0
|
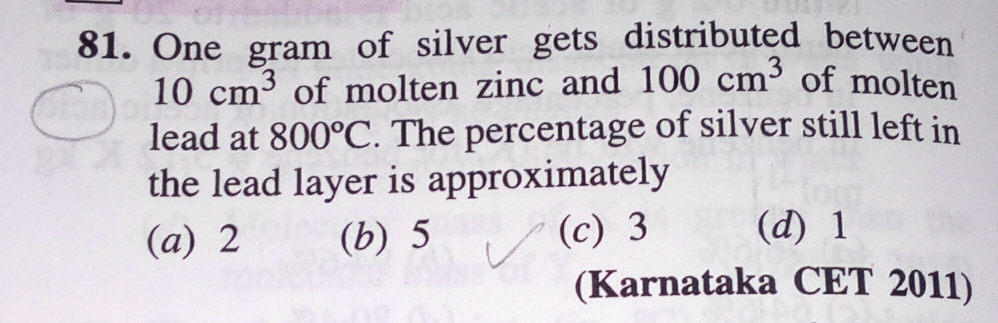
|
Pg 1
|
Terms of Service |
Privacy Policy |
Contact: info@tinkutara.com |